Introduction
Biobanks process, store and distribute high-quality and clinically annotated biological materials. Implementing a quality management system (QMS) at biobank can improve biological material and associated data (BMaD). High-quality BMaD is fundamental for research and benefits both human and veterinary patients. The quality of biological material is dependent on diverse factors throughout the life cycle of the samples, from collection to final utilization. By adhering to biobanking conformity assessment standards, they are well-positioned to provide the consistent quality control and assurance required for research. Implementing standardized practices also helps decrease irreproducibility in the study. However, without conformity assessment, users cannot assure compliance. First, second, or third-party attestation methods can accomplish conformity assessment.
Biobanks receive accreditation when biobanking processes and personnel competence are assessed to be in conformance with standard requirements. With the publication of the ISO standard 20387:2018, biobanks gained access to third-party accreditation to this standard. The Cornell Veterinary Biobank (CVB) recognized this advantage and its commitment to providing researchers with high-quality BMaD. American Association for Laboratory Accreditation (A2LA) evaluated CVB in April 2019. It became the first biobank in the world to gain accreditation to an ISO biobanking standard within eight months. This article describes how CVB attained compliance with ISO 20387:2018 and provides a perspective on the challenges and opportunities encountered. The prototype developed was done based on animal samples; no human samples were used.
Materials and Methods
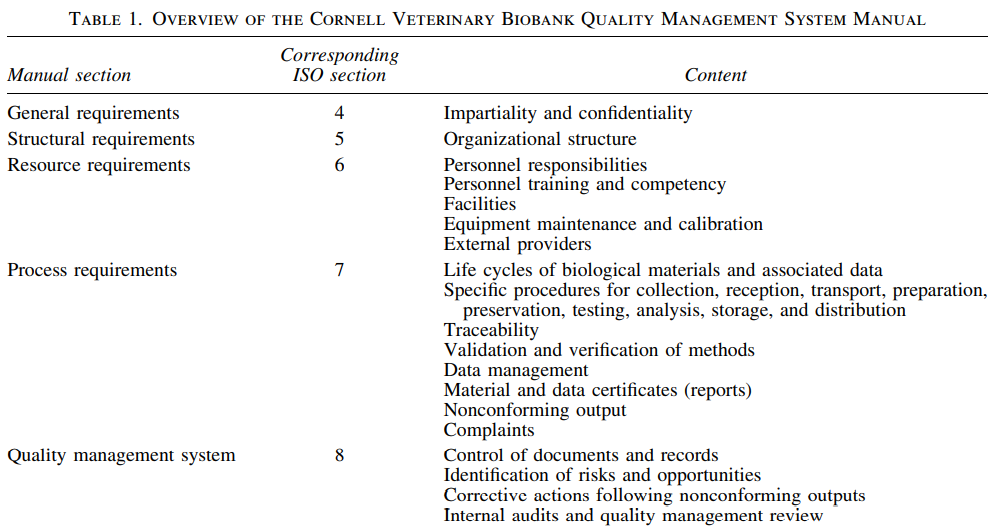
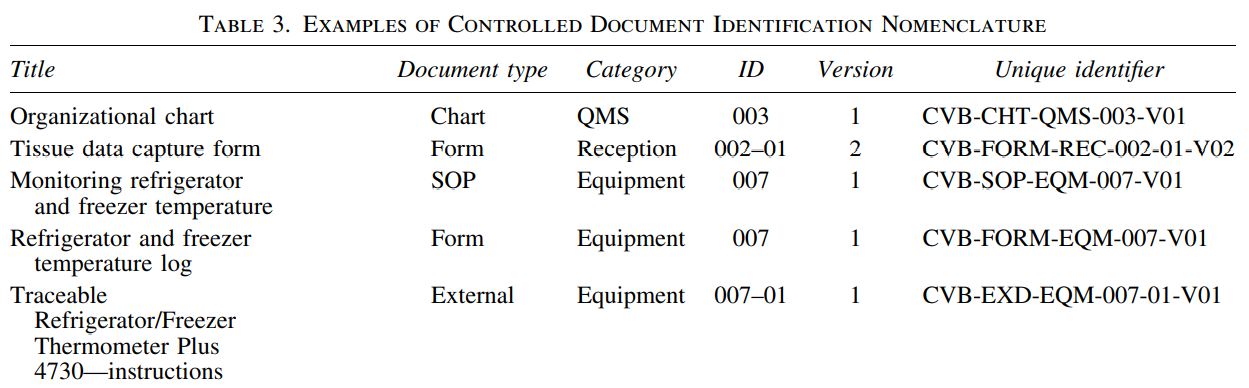
CVB began implementing compliant practices following the publication of ISO 20387:2018. International Society for Biological and Environmental Repositories (ISBER) and National Cancer Institute (NCI) provided a solid foundation for CVB. The first stage of implementation was to establish an intra-university consortium with diverse expertise. A QMS was developed and compiled into a QMS manual. The manual follows ISO standards, with each clause presenting a CVB policy and referencing procedures for each requirement. Procedures relating to needs and detailed documentation of all processes on scope (Table 1) were created. Methods describing the control of documents and records were implemented to provide a foundation to build QMS. It ensured that all documents generated during the creation of the QMS would be uniform within the CVB. CVB personnel prepared for assessment by regularly meeting with the QA manager.
Results
Creating controlled documents and records
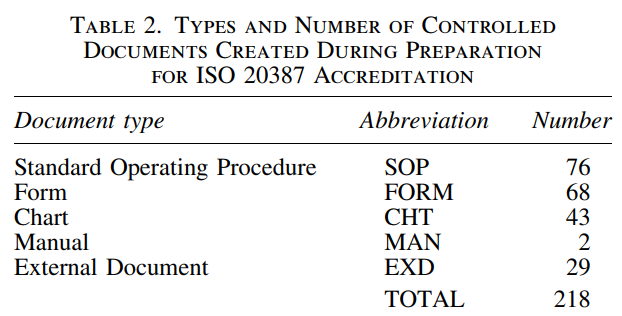
Controlled documents are divided into five types:
- Standard operating procedures (SOPs)- to provide consistent and uniform instructions.
- Forms- to record information related to CVB procedures.
- Charts- for graphical representations or abbreviated summaries of procedures.
- Manuals- as detailed instructions for an entire system.
- External documents- included equipment manufacturer instruction manuals, external submission forms, external standards, and external references for other controlled documents.
A corrective action report (CAR) and action item report were created to document nonconforming output and other events that could adversely affect the quality of CVB products or services. As risks were identified using CARs and action items, these controlled documents were updated, and training events took place to ensure relevant personnel was informed and in compliance.
Governance, Management, and Preparation for Assessment
Documents related to CVB management, structure, equipment, and sample data were created. In addition, the CVB presented the list of proposed materials and processes with corresponding sample life cycle charts, floor plans of CVB facilities, and an entire catalog of banked biological materials. The CVB underwent an assessment visit on April 4 and 5, 2019, consisting of a QMS and technical review. The QMS review aimed to evaluate the evidence of compliance for each clause of the ISO 20387 standard. The technical review aimed to assess the traceability, technical competence, appropriate use of equipment, and accurate description of processes listed on the scope.
After the reviews, the assessor team provided CVB with a list of four deficiencies that needed to be addressed within 40 days for accreditation. A copy of the report and supporting evidence were submitted to A2LA for review upon satisfactory completion of the corrective action. CVB was granted accreditation to ISO 20387 on April 23, 2019, on acceptance of the submitted evidence.
Conclusion
CVB met its accreditation objectives and experienced improvements in operational efficiency, participation from BMaD providers, increased researcher interest, and philanthropy. Another benefit was developing a controlled document system that allows for the rapid identification of required documents while maintaining control of content and distribution. Compliance with ISO 20387 provided unforeseen benefits to CVB’s response to the COVID-19 pandemic.
Accreditation to ISO 20387 can be achieved by all biobanks, including humans, animals, plants, and microorganisms, regardless of size or equipment availability. A review of the ISBER Self-Assessment Tool completed by 62 biobanks between 2015 and 2017 revealed that 43% do not have a QMS in place, suggesting that much progress is needed within the international biobanking community to achieve standardization. As more users/ researchers become educated about the benefits of standardized processes in biobanking, they may request evidence of this compliance. This will encourage biobanks to achieve accreditation to biobanking standards.
