Case Studies
Managing 7 Million Specimens Across Continents: The Scale of Indiana University’s Genetics Biobank Indiana University (IU) manages a large academic biobank that supports over 550
Executive Summary Three of the Netherlands’ largest University Medical Centres—Utrecht, Amsterdam, and Groningen—managed over 9 million biospecimens across 700+ studies using outdated tools like Excel,
The creation of a new Center of Excellence for Modern Biobanking and Biomedical Research (biobank.cy) at the University of Cyprus (UCY) in October 2019 marked
Introduction Telethon Kids (Perth) is one of Australia’s largest medical research institutes with the vision to improve the health and well-being of children through excellence
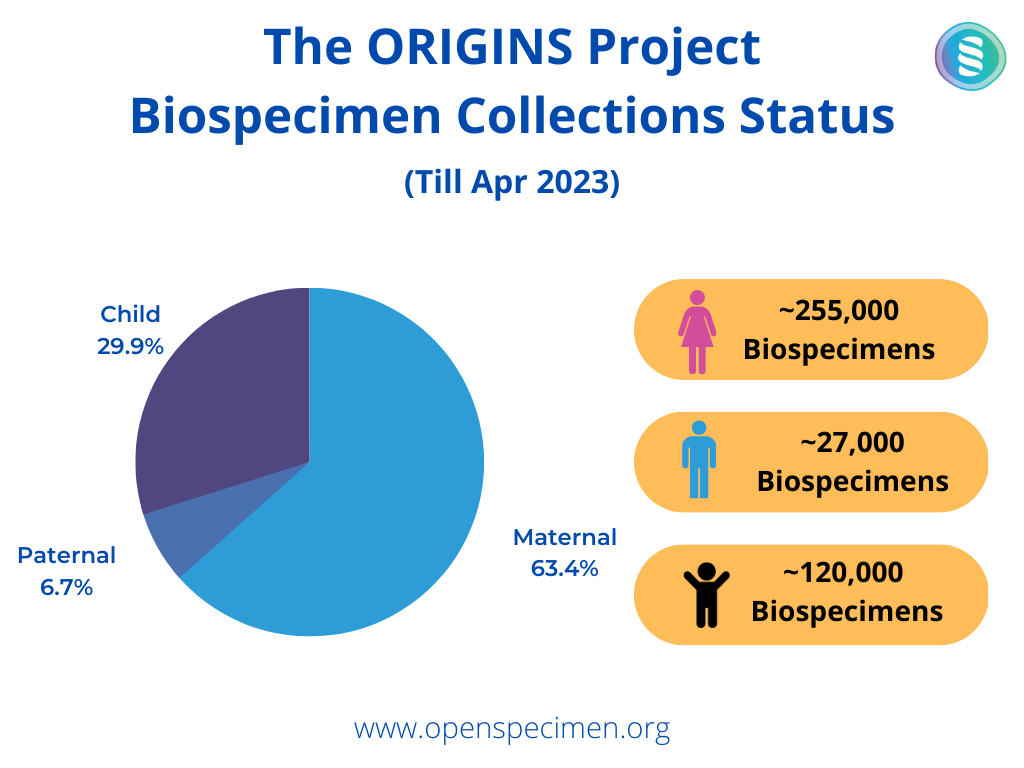
The “PREgnancy Care Integrating translational Science, Everywhere” (PRECISE) Network aims to develop a unique cohort of biologically and contextually characterized pregnant and non-pregnant women of
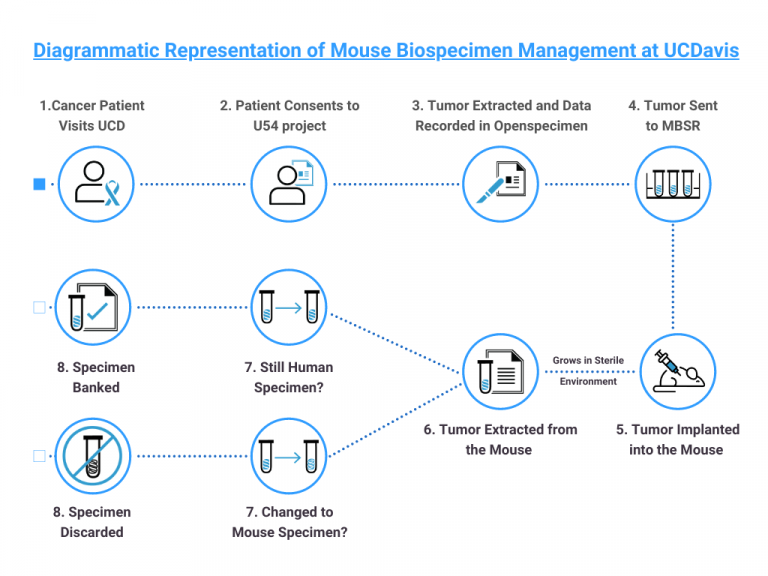
The Mouse Biology Program (MBP) of the University of California, Davis (UCD) is the largest academic program in the world. MBP is a member of the NIH-funded Mutant Mouse Resource
The Victorian Cancer Biobank (VCB) is a crucial part of the medical research landscape supporting researchers throughout Australia and around the world with the aim of delivering
Currently, biobanks at the University Hospital Basel are not centralized. Storage locations are distributed over organizational units, which partly are about to be centrally managed. Biobank inventory
The Winship Cancer Institute Clinical Trial office (CTO) conducts high-quality clinical trials involving cancer patients. It conducts over 250 clinical trials enrolling roughly 800 patients per year. In
Research in precision medicine requires a continuous supply of high-quality biospecimens. But as the saying goes- “Garbage in garbage out”, meaningful conclusions from the studies