The enthusiasm surrounding biobanking and clinical research has gone through an upsurge in the past decade. Governments and industries have been heavily investing in these studies and, undoubtedly, the medical and scientific benefits have been enormous. But even with such scale of infrastructure, the industry lacks a consensus on the subject of ‘Informed consent’ (1,2).
OpenSpecimen allows the tracking of the consent of the participants involved in a study. The management of consent involves:
- Managing consent statements.
- Setting up consent statements for a study.
- Capturing the participant’s consent responses.
- Ensuring that the consent-related obligations are followed during distribution.
Creating consent statements
Administrators can add consent statements along with a unique code for easy identification. The system checks and prevents duplication of consent statements and their codes. The consent statements are standardized and can be used for multiple studies.
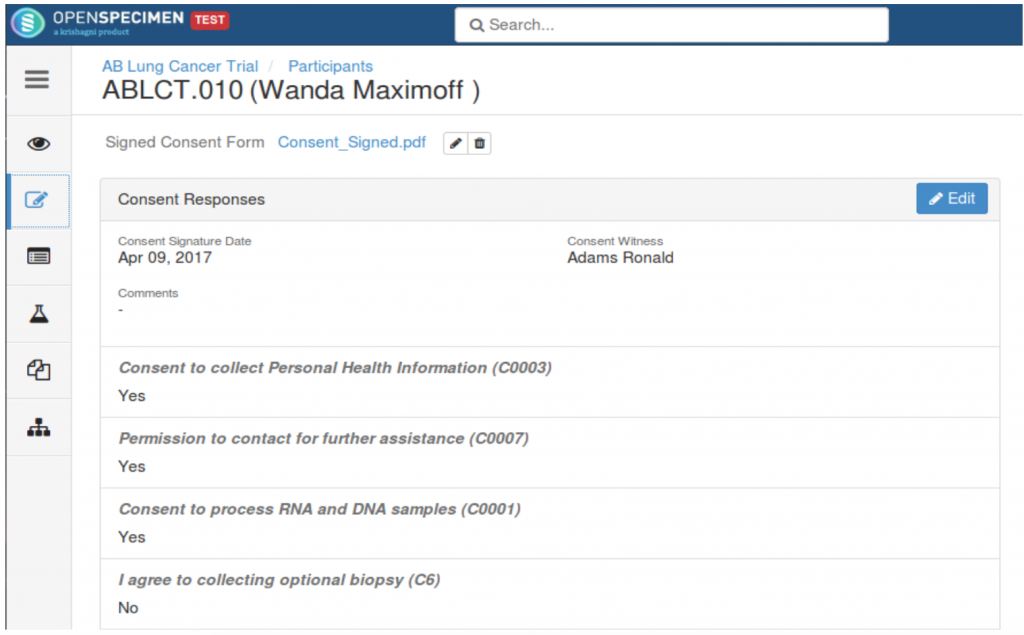
Capturing participant responses
One of the many interesting features of the software is the designing of a longitudinal study with specific time points, to which, a user can pre-assign the consent statements and the response of participants can also be captured. To make the process easier for studies involving many participants, OpenSpecimen allows feeding data in bulk. There is also a feature to upload a signed consent form.
Specimen distribution
It’s the responsibility of biobanks involved in specimen distribution to ensure that the distributed specimen is not used for any purpose to which the participant has not given consent. OpenSpecimen allows users to add consent statements in distribution studies as well, which the system compares against the consent linked to the specimen to be distributed and restricts the distribution in case the consents don’t match.
Reporting
OpenSpecimen allows users to query for specimens based on participant consents data. For e.g. Search for DNA specimens from participants who have consented for ‘Use of DNA specimens for research’ or Search for participants who have become 18 in order to re-consent.
Summary
OpenSpecimen simplifies the overall consent management process for a biobank by providing an easy to use platform with multiple features. It helps the biobank not only for efficient management but also in gaining public trust.
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5526496/
- https://www.fda.gov/ForPatients/ClinicalTrials/InformedConsent/ucm20041763.htm
Written by: Poornima Govindrao, Product Manager, Krishagni
For more details, email [email protected]
