Research in precision medicine requires a continuous supply of high-quality biospecimens. But as the saying goes- “Garbage in garbage out”, meaningful conclusions from the studies can only be drawn when the researchers are assured that the materials used in their research were of good quality.
A survey(1) conducted by the National Cancer Institute (NCI) funded cancer researchers, showed that lack of quality biospecimens resulted in 60% of researchers questioning their findings and 81% limiting the scope of their work.
In our second webinar, Ms. Irmi Feldman, Manager of the University of California Davis Pathology Biorepository, talked about how they prepared for and achieved CAP accreditation. In this article, we will discuss some of the highlights from the webinar.
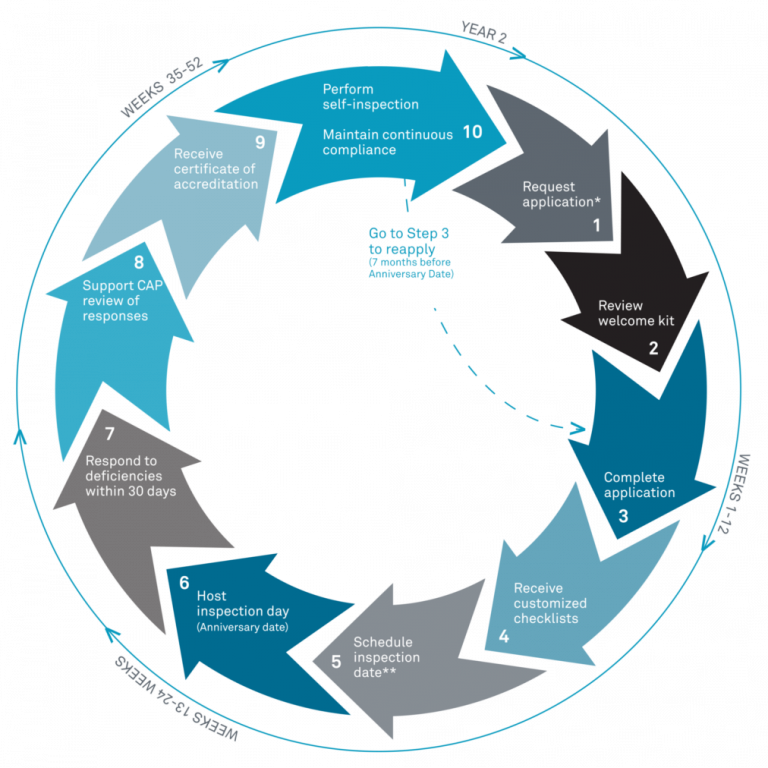
Accreditation Cycle
In fall 2019, CAP changed the accreditation cycle for Biorepositories from three years to two years to align biorepository checklists with the checklists of CLIA-approved laboratories to allow potential usage of biorepository specimens for patient care. To apply for the CAP BAP program, one has to submit an application via the CAP website (cap.org) including an enrolment fee, and the biorepository’s activity menu(2). CAP will provide customized checklists based on your biorepository’s activity menu.
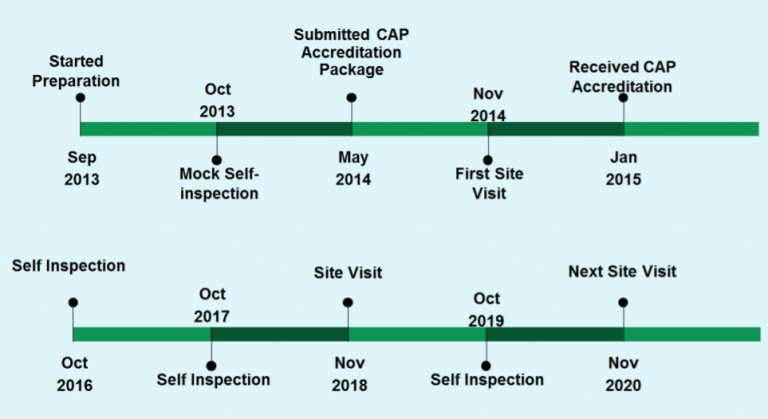
The UCD Pathology Biorepository received its CAP accreditation in January 2015. The first CAP inspection was due in November 2014 and its preparations began in June 2013 followed by a Mock Self-Inspection in Oct 2013.
Inspection teams are selected from peer institutions based on common areas of activity. They are comprised of pathologists, Clinical Lab and biorepository managers. CAP inspectors undergo formal training by CAP in the use of the R.O.A.D. technique (Read, Observe, Ask, Discover).
Preparatory steps
After submitting the initial application packet, CAP provides customized checklists for BAP, GEN, COM, and DRA based on the activities identified in the original application. The checklist acts as a guide for the preparatory steps. The following are some insights into how the UCD team prepared for the inspection.
Policies and Procedures
The UCD team developed Policies & Procedures (P&Ps) based on the individual items on the CAP checklists. They divided P&Ps into sections such as storage, IT, Informed Consent & IRB, safety procedures, equipment, specimen processing, distribution, research histology, and Biorepository Admin. To ensure that all items on the CAP checklists are addressed in the P&Ps, they created a list cross-referencing both. Also, they track P&Ps (date of adoption and date of retirement) in a Document Control System as required by CAP.
Quality Management
Creating a Quality Management Program with specific quality indicators is crucial to the successful execution of biorepository workflows and practices. It ensures that the biorepositories follow the standardized procedures when they procure, process, store, and distribute the specimens. Further, the procedures include Chemical Safety Plans, general safety guidelines, and initiation of a Biospecimen Utilization Committee as well as quarterly quality meetings.
Personnel Training
For personnel’s training, they created detailed staff training modules for all positions. It involved initial training sessions followed by competency assessment sessions and quizzes. They tracked all staff training in OpenSpecimen– which they use as their biobanking LIMS.
Benefits of CAP Accreditation
CAP accreditation ensures that the specimens that a biorepository collects, processes, stores, and distributes to researchers are of the highest quality. The accreditation cycle is providing a means to maintain standardization. In addition, it also promotes an ongoing commitment to follow NCI’s Best Practices for biospecimen management.
In addition, Biorepository accreditation helps to ensure:
- Appropriate ethical and legal frameworks for the use of biospecimens
- Well-controlled pre-analytic variables
- Robust chain of custody tracking (reduced risk of misidentification)
- Appropriate storage conditions and temperature monitoring
- Best practice policies and procedures for sample collection/processing/release
- Histologic quality assurance
- Confidence in the long-term quality of biospecimens
- Improved grant/funding opportunities
References:
- Assessing the need for a standardized cancer Human Biobank (caHUB): findings from a national survey with cancer researchers. Massett HA1, Atkinson NL, Weber D, Myles R, Ryan C, Grady M, Compton C.
- https://www.cap.org/laboratory-improvement/accreditation/biorepository-accreditation-program
