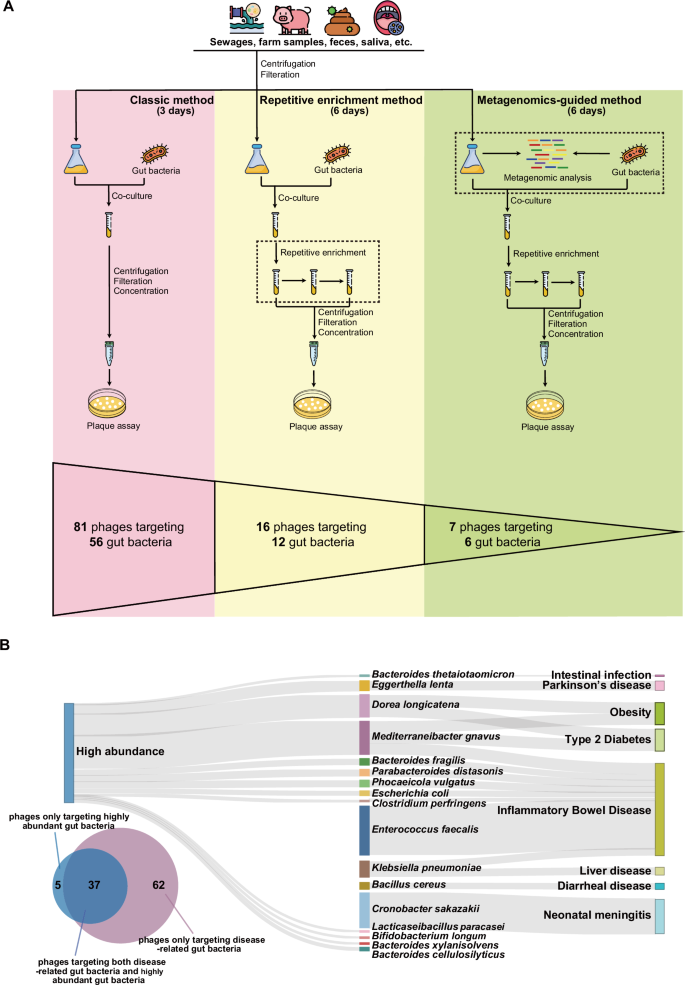
Abstract: For years, biobanks have focused on cells, tissues, and fluids we can easily name. But a new kind of repository is quietly expanding the definition of what’s worth preserving. The Gut Phage Biobank brings bacteriophage viruses that infect bacteria into the spotlight, capturing entities that shape our microbiome without ever being seen. By systematically isolating and cataloguing gut phages that target disease-associated and abundant bacteria, researchers are turning microbial “dark matter” into structured, usable knowledge. This isn’t just about storage; it’s about creating order in one of the most complex ecosystems in the human body. A biobank like this becomes a bridge between raw biology and actionable science. It allows researchers to move from observation to intervention. And it shows how biobanking is evolving beyond samples into systems.
What makes this effort compelling is how it connects infrastructure with possibility. By combining cultivation workflows, genomic analysis, and global prevalence data, the Gut Phage Biobank becomes a living reference for microbiome research. These phages aren’t archived in isolation they’re linked to host specificity, geography, disease states, and therapeutic potential. In a space where most gut viruses remain uncultured, this biobank offers something rare: control and clarity. It opens doors to precision microbiome modulation, where phages could complement probiotics or even replace blunt interventions. More importantly, it reframes biobanks as engines of discovery, not endpoints. Sometimes, the smallest things stored can unlock the biggest shifts in how we treat disease.
Read this interesting article here: https://www.nature.com/articles/s41467-025-61946-0